Wholesale China Cross Linked Hyaluronic Acid Fillers Factories Exporter – Dermaxgel Modified Sodium Hyaluronate gel dermal fillers CE2292 – FLODERMA
Wholesale China Cross Linked Hyaluronic Acid Fillers Factories Exporter – Dermaxgel Modified Sodium Hyaluronate gel dermal fillers CE2292 – FLODERMA Detail:
What does Dermaxgel™ do?
Dermaxgel™ injectable gel temporarily adds volume to facial tissue and restores a smoother appearance to the face.
Trade name:Dermaxgel TM
Generic name:Modified Sodium Hyaluronate Gel For Injection
| Description | Values | ||
| Model | Deep | Deep | Deep |
| Specifications | 1ml | 2ml | 10ml |
| Gel Size | 0.28-0.50(mm) | 0.28-0.50(mm) | 0.28-0.50(mm) |
| Needle size | 27G*2 | 27G*2 | Not supplied |
| Duration | From 6-12 months | From 6-12 months | From 6-12 months |
| concentration | From 20mg-30mg/ml | From 20mg-30mg/ml | From 20mg-30mg/ml |
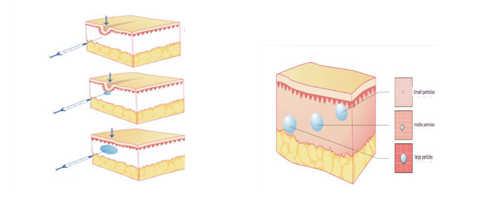
| Where to inject | Deep layer of dermis and/of surface layer of subcutis | ||
| Recommended indications | Derm facial wrinkles and folds, like the nasolabial folds,shaping facialcontours, e.g cheeks fullness, chin and lips augmentation. |
||
CONTENT
Stabilized hyaluronic acid = 20mg/ml; Sodium chloride physiological solution q.s; PH 7
DESCRIPTION
It is a sterile pyrogen-free physiological gel of crosslinked hyaluronic acid, of non-animal origin.
It is a colorless, odorless and highly viscous aqueous gel. It is supplied in a glass syringe with a luer-lock fitting. The contents of the syringe.Have been sterilized using moist heat. The product is for single use only. Disposable sterile Needles are provided with each syringe.Information about the sterilization method and size of the needle is printed on its packaging. The number of units per package and the volume contained in each syringe is as started on the outer package.
INTENDED USE
It is intended to be used for intradermal implantation to correct defect of soft tissue after accidents and trauma.
STERILIZATION
The syringe containing is sterilized by steam. The needle is sterilized by means of gama radiation
MODE OF ACTION
This product is a filler that adds volume to the tissue. The volume and the lifting capacity originate from the ability of.The hyaluronic acid to attract high amount of water, Which is further increased by the stabilization process.It will in time undergo isovolemic degradation, Which means that the product maintains its Volume even during degradation.
INDICATION
It is indicated for soft tissue augmentation. The depth of injection is the dermal layer of the skin.For facial areas with limited soft tissue support and soft tissue cover,e.g.the periorbital Region, injection into the subcutaneous fatty tissue or supraperiostal administration are recommended.
Product detail pictures:









Related Product Guide:
We consistently execute our spirit of ''Innovation bringing progress, Highly-quality ensuring subsistence, Administration advertising and marketing gain, Credit history attracting buyers for Wholesale China Cross Linked Hyaluronic Acid Fillers Factories Exporter – Dermaxgel Modified Sodium Hyaluronate gel dermal fillers CE2292 – FLODERMA , The product will supply to all over the world, such as: Roman, Portugal, Vietnam, There are advanced producing & processing equipment and skilled workers to ensure the merchandise with high quality. We've got found an excellent before-sale, sale, after-sale service to ensure the customers that could rest assured to make orders. Until now our merchandise are now moving on fast and very popular in South America, East Asia, the Middle east, Africa, etc.
Hope that the company could stick to the enterprise spirit of "Quality, Efficiency, Innovation and Integrity", it will be better and better in the future.